Interfacial Charge Transfer from Molecules to Metals
 Optically induced charge transfer between adsorbed molecules and a
metal or semiconductor electrode has been explored. The absorption of a redox couple on the surface
of an electrode was predicted by Hush to lead to new electronic
absorption features, but has not been experimentally observed. However,
Gerischer characterized photocurrents arising from such absorption
between adsorbed metal atoms and semiconductor conduction bands.
Optically induced charge transfer between adsorbed molecules and a
metal or semiconductor electrode has been explored. The absorption of a redox couple on the surface
of an electrode was predicted by Hush to lead to new electronic
absorption features, but has not been experimentally observed. However,
Gerischer characterized photocurrents arising from such absorption
between adsorbed metal atoms and semiconductor conduction bands.
Interfacial charge transfer absorption (IFCTA) provides information
concerning the barriers to charge transfer between molecules and the
metal/semiconductor and the magnitude of the electronic coupling and
could thus provide a powerful tool for understanding interfacial
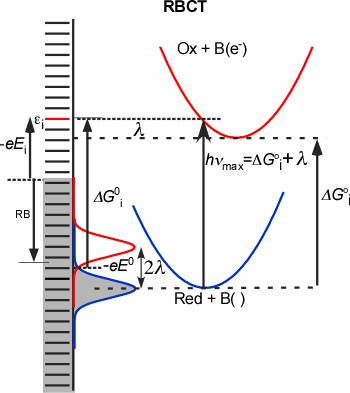
charge-transfer kinetics. We have developed a framework for modeling and
predicting IFCTA spectra. The key feature of optical charge-transfer to
or from a band of electronic levels (taken to have a constant density of
states and electronic coupling element) is that the absorption
probability reaches half intensity at λ + ΔG0 , where λ and ΔG0 are the
reorganization energy and free energy gap for the optical charge
transfer, attains >90% intensity at λ + ΔG0 + 0.9(4λkT)1/2 and remains essentially constant
until the top (bottom) level of the band is attained. However, when the
electronic coupling and transition moment are assumed independent of
photon energy (Mulliken-Hush model), a peaked, highly asymmetric
absorption profile is predicted.
We conclude that, in general, the
electronic coupling between molecular adsorbates and the metal levels is
so small that absorption is not detectable, whereas, for semiconductors,
there may be intense features involving coupling to surface states.

 Optically induced charge transfer between adsorbed molecules and a
metal or semiconductor electrode has been explored. The absorption of a redox couple on the surface
of an electrode was predicted by Hush to lead to new electronic
absorption features, but has not been experimentally observed. However,
Gerischer characterized photocurrents arising from such absorption
between adsorbed metal atoms and semiconductor conduction bands.
Optically induced charge transfer between adsorbed molecules and a
metal or semiconductor electrode has been explored. The absorption of a redox couple on the surface
of an electrode was predicted by Hush to lead to new electronic
absorption features, but has not been experimentally observed. However,
Gerischer characterized photocurrents arising from such absorption
between adsorbed metal atoms and semiconductor conduction bands.