Solar Photoconversion
The amount of energy striking the earth from sunlight in one hour is equal to all the energy consumed globally from fossil fuels in a year.
Thus the solar flux provides an enormous source of renewable energy; however, its economic use requires a stable method to store and distribute the energy.
The only scalable and efficient method to store solar energy is by its conversion into chemical bonds.
Thus, the use of sunlight to split water, or artificial photosynthesis, is the Holy Grail of renewable energy research.
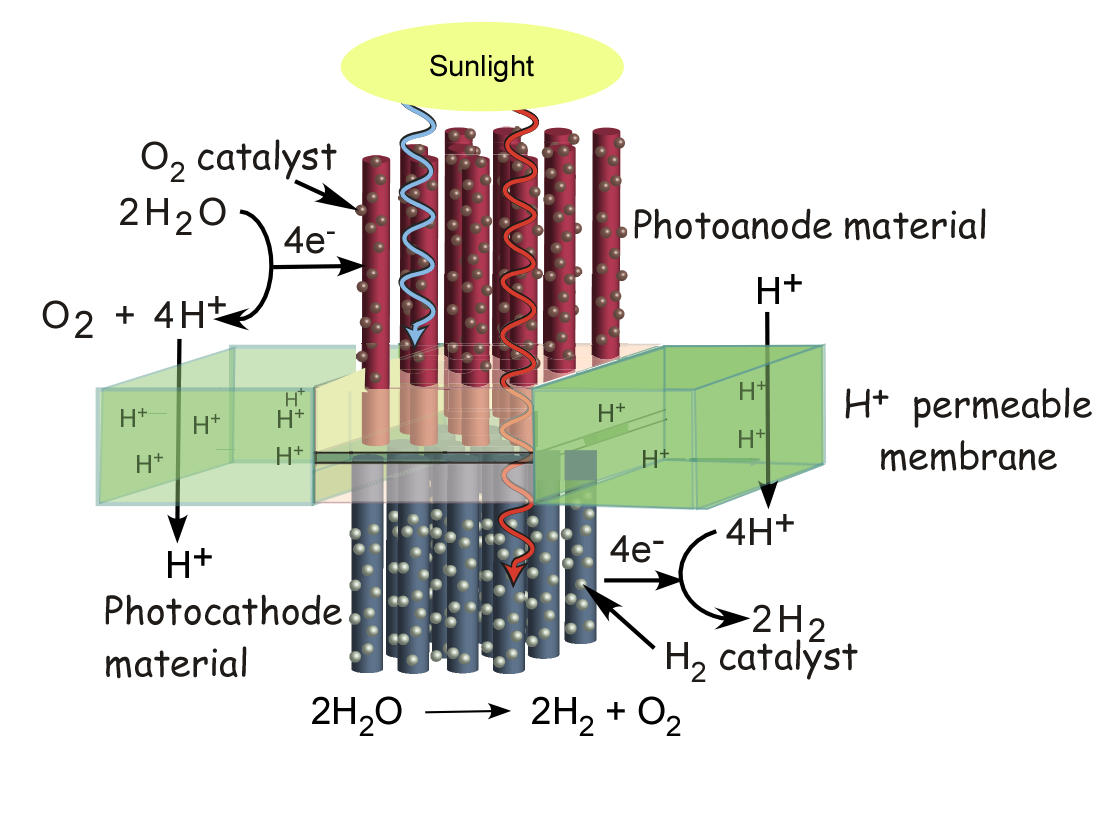
We are pursuing a water-splitting photoconversion system that will produce hydrogen fuel directly from water using only inexpensive materials.
Conceptually our cell is built around a semiconductor membrane that converts sunlight into electron/hole pairs.
The photogenerated electrons and holes would be directed to opposite sides of the membrane where catalysts in contact with a water solution would produce hydrogen and oxygen.
Fundamentally the materials must be able to generate the 2.46 eV of free energy needed to split water into H2 and 1/2 O2.
The cell will be constructed of two semiconductors as light absorbers imbedded in an electrical and ion conduction membrane.
The membrane will separate the cell into two compartments where either the water oxidation or reduction reactions will occur.
The absorbers will be made from cheap polycrystalline semiconductors and will separate the electrons and holes across the liquid/semiconductor junction.
In order to achieve efficient charge separation the semiconductors will be formed into a micro/nano-rod assembly with catalysts for
water reduction and oxidation attached to the sides of the semiconductors. Oxidation and reduction will occur on opposite sides of the membrane,
thus requiring each semiconductor to be robust to a single reaction and the use of two different catalysts. Finally, the system will make use of two
solar photons to provide the energy to drive the reactions.
The development of a solar photoconversion device will require catalyst develop, studies of the kinetics and mechanism of hydrogen and oxygen generation,
knowledge of how to attach active catalysts to semiconductor surfaces, semiconductor development as well as membrane construction.
Fundamental work is being pursued in all of the above areas.

